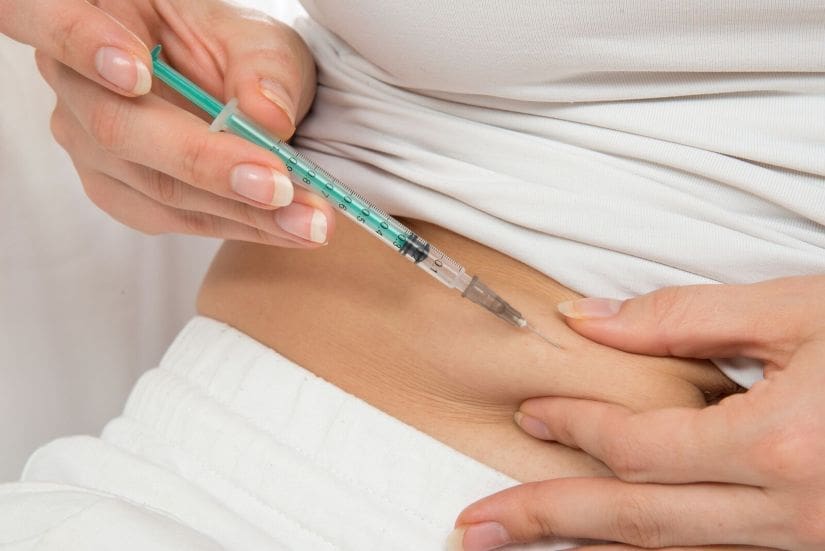
If you are about to undergo an assisted reproduction treatment, you may have questions about the medication you will have to take and the side effects it may have on your body.
Some of the medications used in in vitro fertilization treatments can cause abdominal discomfort and bloating, nausea, vomiting, dizziness and mood alterations. Many of these effects are mild and similar to those caused by the hormonal changes of the menstrual cycle or pregnancy.
Below, we talk about the main medications used in IVF treatments and we focus on one of the most common ones: Decapeptyl.
IVF treatment medications
Almost all the medications used in IVF are intended to stimulate, stop or substitute the hormones involved in ovulation.
Most of them can be self-administered at home by taking oral tablets, vaginal or subcutaneous injections, according to your doctor’s instructions.
IVF treatment medication and its dosage depends on each woman, although there are some commonly used drugs.
Among them:
- Gonadotropins. These hormones are naturally involved in follicular development. Their administration allows controlled stimulation of ovarian function.
- Oral contraceptives. In some patients, contraceptives help control the menstrual cycle and allow a better response to medication.
- Clomiphene citrate. Combined with other drugs, it can induce ovulation.
- Gonadotropin-releasing hormone (GnRH) agonists. They can be administered at different times of the IVF cycle. Their purpose is to enhance the gonadotropin response. Decapeptyl is one of the most popular GnRH agonists.
- GnRH antagonists. They help prevent early ovulation to successfully complete IVF treatment.
- Progesterone. Provides hormonal support to the luteal phase of the cycle, just after ovulation. This hormone is only administered when the patient does not produce it in sufficient quantity or when the treatment requires it.
- Estrogens. They prepare the endometrium and make it more “receptive” to embryo implantation.
What is Decapeptyl?
Decapeptyl is a hormonal drug commonly used in IVF treatments. Its active ingredient is triptorelin, a synthetic extended-release decapeptide analog of gonadotropin-releasing hormone.
This basically means that it acts the same way as gonadotropin-releasing hormone. However, triptorelin has an “advantage” over its natural counterpart in that it stimulates the synthesis and release of FSH (luteinizing hormone) and LH (follicle stimulating hormone) more effectively and for a longer period of time.
The initial increase of these hormones causes an increase in the production of estrogen in the ovaries and testosterone in the testes, which in turn inhibits the hypothalamic production of GnRH, by negative feed-back, feeding back the hypothalamic-pituitary-gonadal axis (i.e., in a second phase it produces an inhibition of the release of these hormones by desensitization of the receptors).
In general terms, triptorelin makes it possible to control ovarian function throughout ovarian stimulation. By administering Decapeptyl to the patient, physicians can control the LH surge that triggers ovulation, thus avoiding a possible spontaneous expulsion of the egg, which would force the cancellation of the IVF cycle.
Applications of Decapeptyl
Decapeptyl is one of the most common drugs used to treat female infertility and is used as an adjunctive treatment to gonadotropins (HMG, FSH, hCG) during the ovarian stimulation phase of IVF.
Decapeptyl is indicated to treat female infertility. Its use is contemplated in women with a history of adverse IVF results, with premature LH peaks or premature luteinization, with production of poor quality oocytes.
In addition, it also enables monitoring of ovarian activity in women undergoing embryo transfer with frozen embryos or in oocyte donation cycles. It facilitates the control of endometrial growth and synchronization in oocyte donation cycles.
It is also indicated in other pathologies such as endometriosis, myomas, precocious puberty and some cancers.
Decapeptyl before oocyte puncture as an ovulation trigger
Applied as a bolus (intravenous), it induces the release of FSH and LH in 24-36 hours, causing the maturation and release of the oocyte, ready to be extracted in the puncture, but it causes an insufficient luteal phase, having to postpone the transfer in the case of patients who want to do it fresh.
Its use to prevent ovarian hyperstimulation syndrome (OHSS) is a widespread strategy to induce oocyte maturation and ovulation when a protocol with antagonists (ant-GnRH) has been used previously.
Its use in donors eliminates the risk of OHSS without affecting the outcome in recipients.
Decapeptyl after surgeries or when you want to “stop” cycles.
It produces pituitary suppression and, therefore, blockage of the ovarian cycle and endometrial atrophy. For this reason it is used after endometriosis surgery. Its use prolongs the pain-free period after surgery and does not affect the reproductive prognosis.
As this drug allows to control the activity of the ovaries, it is widely used in women who are going to undergo embryo transfer of frozen embryos or oocyte donation treatments, as it allows to control the growth of the endometrium and helps to synchronize the cycles between donor and recipient in oocyte donation treatments.
Administration of Decapeptyl
Decapeptyl is available in four forms: daily (0.1mg/1ml), monthly (3.75mg), quarterly (11.25mg) or semi-annually (22.5mg). In addition, it can be administered parenterally subcutaneously, by inhalation and intramuscularly.
One or the other is prescribed depending on the medical strategy. In general, decapeptyl is used daily or monthly in IVF treatments. If a monthly injection is given with menstruation, a new period is expected after 4-5 weeks, but sometimes it can be delayed.
The 3 month formulation is used in certain surgeries or situations where it is necessary to control and stop the menstrual cycle for a longer time, causing a temporary interruption of the menstrual period.
The active ingredient is Triptorelin and its excipients are mannitol and sodium chloride, and the preparations contain water as a solvent for mixing with the powder.
It can be stored at room temperature (Tª < 25ºC), protected from light.
Does Decapeptyl have any side effects?
Decapeptyl does not usually present significant side effects in its use for assisted reproduction treatments.
The main side effects are a consequence of changes in hormone levels.
The main limitation of this drug is the occurrence of side effects due to hypoestrogenism (decrease in estrogen), especially at the bone level.
For this reason, treatments should be short or supplemented with hormone replacement therapy (add-back therapy) with low doses of estrogens to avoid the symptoms of hypoestrogenism without stimulating the progression of endometriosis.
It can produce vulvovaginal dryness and pain during intercourse, erythema, pain and inflammation at the injection site, dizziness, headache, mood swings, sweating, hot flashes, abdominal pain, nausea, asthenia or fatigue.
Prolonged use increases the risk of osteoporosis in patients with risk factors (alcohol consumption, smoking, malnutrition, use of other drugs that reduce bone density).
Another side effect of Decapeptyl injections involves skin irritation, so we recommend that you change the puncture site every day. In Juana Crespo we are experts in assisted reproduction. Do you want information about our IVF treatments? Get in touch with us.